
Thus in the 50.5 days it takes half the 89Sr atoms to decay, emitting the same number of beta particles as there were decays, less than 0.4% of the 90Sr atoms have decayed, emitting only 0.4% of the betas. But 90Sr has a 30-year half-life, and 89Sr a 50.5-day half-life. For instance, strontium-89 and strontium-90 are produced in similar quantities in fission, and each nucleus decays by beta emission. The produced radionuclides have varying half-lives, and therefore vary in radioactivity. Thus, fission events normally result in beta and gamma radiation, even though this radiation is not produced directly by the fission event itself. This releases additional energy in the form of beta particles, antineutrinos, and gamma rays. Due to being relatively neutron-rich for their atomic number, many of them quickly undergo beta decay.
:max_bytes(150000):strip_icc()/fission-of-a-uranium-nucleus-141483757-579266b03df78c173498d794.jpg)
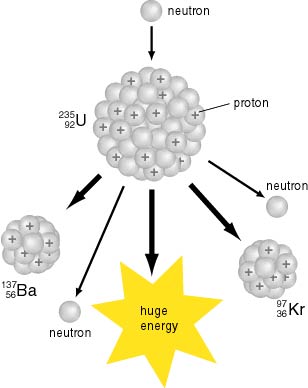
The fission products themselves are usually unstable and therefore radioactive. (See also Fission products (by element)).Ībout 0.2% to 0.4% of fissions are ternary fissions, producing a third light nucleus such as helium-4 (90%) or tritium (7%). The two smaller nuclei are the fission products. Typically, a large nucleus like that of uranium fissions by splitting into two smaller nuclei, along with a few neutrons, the release of heat energy ( kinetic energy of the nuclei), and gamma rays. Nuclear fission products are the atomic fragments left after a large atomic nucleus undergoes nuclear fission.


 0 kommentar(er)
0 kommentar(er)
